Buffer-gas trap
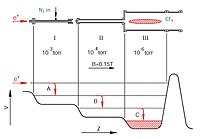
A buffer-gas trap is like a special container that holds tiny particles like atoms or molecules. Imagine that you have a toy box that can only hold very small toys, like tiny Lego pieces. You want to keep them all in one place so they don't scatter around and get lost. The buffer-gas trap works in a similar way, but instead of toys, it holds particles that scientists want to study.
The trap has two parts. The first part is a vacuum chamber, which means that there is no air inside. The second part is a container filled with a gas, like helium or neon, which we can call the "buffer gas". When the particles are released into the trap, they bounce around and bump into the buffer gas. Every time they collide with the buffer gas, they lose a little bit of energy and slow down.
The buffer gas helps to cool down the particles by transferring some of the kinetic energy (energy of motion) from the particles to itself. As the particles slow down, they become easier to study because they stay in one place and don't move around as much. It's like trying to read a book while a bunch of bees are flying around you - it's hard to focus! But if you put the bees in a jar, it's much easier to read the book.
The buffer-gas trap is important for scientists because it allows them to study the properties of particles that are too small to see with the naked eye. By trapping these particles and slowing them down, researchers can make very precise measurements of their behavior and properties. This helps scientists understand theories about how the world works on a very small scale, which can lead to new technologies and discoveries in fields like medicine, energy, and materials science.
The trap has two parts. The first part is a vacuum chamber, which means that there is no air inside. The second part is a container filled with a gas, like helium or neon, which we can call the "buffer gas". When the particles are released into the trap, they bounce around and bump into the buffer gas. Every time they collide with the buffer gas, they lose a little bit of energy and slow down.
The buffer gas helps to cool down the particles by transferring some of the kinetic energy (energy of motion) from the particles to itself. As the particles slow down, they become easier to study because they stay in one place and don't move around as much. It's like trying to read a book while a bunch of bees are flying around you - it's hard to focus! But if you put the bees in a jar, it's much easier to read the book.
The buffer-gas trap is important for scientists because it allows them to study the properties of particles that are too small to see with the naked eye. By trapping these particles and slowing them down, researchers can make very precise measurements of their behavior and properties. This helps scientists understand theories about how the world works on a very small scale, which can lead to new technologies and discoveries in fields like medicine, energy, and materials science.
Related topics others have asked about:
