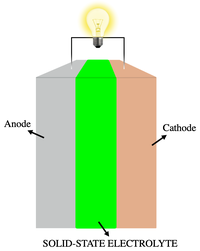
Solid-state electrolyte
Imagine you have two pieces of candy that you want to touch together. The candy pieces need to be connected so that they can share their sweetness. But if you tried to connect them with a piece of string, it would break easily and the candy wouldn't be able to touch. That's why you need something, like a magical glue, that can hold the candy together without breaking.
In the world of science, we use something a bit like magical glue called an electrolyte, which helps substances connect, or conduct, with each other. An electrolyte is like a bridge, helping charged particles called ions move from one place to another. Without an electrolyte, the ions would be stuck in one place and wouldn't be able to move.
Now, a solid-state electrolyte is a special kind of electrolyte that is like a candy that can't melt. Usually, electrolytes are liquids or gels, but a solid-state electrolyte is like a hard candy that can conduct electricity. Imagine a candy that is so hard that you can't bite through it, but still has sweetness inside. Now imagine that this candy is also able to pass electricity through it. That's what a solid-state electrolyte is like.
Solid-state electrolytes have many advantages over liquid electrolytes. For one thing, they are safer because they don't leak or spill like liquid ones do. They are also more stable and can operate at higher temperatures, which makes them useful in things like batteries for electric cars. They are also more efficient because they have less resistance to the flow of electricity, which means they waste less energy.
So, to sum it up, a solid-state electrolyte is like a hard candy that can conduct electricity. It's a special kind of glue that helps charged particles move from one place to another, and it's important because it can make things like batteries better and safer.
In the world of science, we use something a bit like magical glue called an electrolyte, which helps substances connect, or conduct, with each other. An electrolyte is like a bridge, helping charged particles called ions move from one place to another. Without an electrolyte, the ions would be stuck in one place and wouldn't be able to move.
Now, a solid-state electrolyte is a special kind of electrolyte that is like a candy that can't melt. Usually, electrolytes are liquids or gels, but a solid-state electrolyte is like a hard candy that can conduct electricity. Imagine a candy that is so hard that you can't bite through it, but still has sweetness inside. Now imagine that this candy is also able to pass electricity through it. That's what a solid-state electrolyte is like.
Solid-state electrolytes have many advantages over liquid electrolytes. For one thing, they are safer because they don't leak or spill like liquid ones do. They are also more stable and can operate at higher temperatures, which makes them useful in things like batteries for electric cars. They are also more efficient because they have less resistance to the flow of electricity, which means they waste less energy.
So, to sum it up, a solid-state electrolyte is like a hard candy that can conduct electricity. It's a special kind of glue that helps charged particles move from one place to another, and it's important because it can make things like batteries better and safer.
Related topics others have asked about:
