Acid catalysis
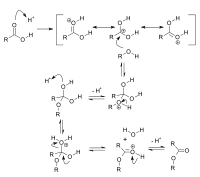
Okay kiddo, let's talk about acid catalysis.
Do you know what acid is? Some things taste sour and that's because they're acidic. But acid is more than just what we taste. It's a chemical that donates a hydrogen ion, also known as a proton.
Now, when we say "catalysis," we mean that something helps a chemical reaction happen faster. It's like a helper that makes things go smoother and quicker.
So, when we put acid in a chemical reaction, it helps make things happen faster. But how does it do that? Acid catalysis works by making the reaction more favorable. It lowers the energy needed to start the reaction (also known as the activation energy).
Think of it like how it's easier to ride a bike downhill than uphill. Acid catalysis helps make the reaction go downhill, which means it needs less energy overall.
But acid catalysis isn't just about making reactions happen faster. It can also change the outcome of the reaction. Sometimes, acid catalysis can create new molecules that wouldn't have been formed without the acid being present.
Overall, acid catalysis is like a cheerleader for chemical reactions. It gives them the boost they need to happen and can create all sorts of new and exciting products.
Do you know what acid is? Some things taste sour and that's because they're acidic. But acid is more than just what we taste. It's a chemical that donates a hydrogen ion, also known as a proton.
Now, when we say "catalysis," we mean that something helps a chemical reaction happen faster. It's like a helper that makes things go smoother and quicker.
So, when we put acid in a chemical reaction, it helps make things happen faster. But how does it do that? Acid catalysis works by making the reaction more favorable. It lowers the energy needed to start the reaction (also known as the activation energy).
Think of it like how it's easier to ride a bike downhill than uphill. Acid catalysis helps make the reaction go downhill, which means it needs less energy overall.
But acid catalysis isn't just about making reactions happen faster. It can also change the outcome of the reaction. Sometimes, acid catalysis can create new molecules that wouldn't have been formed without the acid being present.
Overall, acid catalysis is like a cheerleader for chemical reactions. It gives them the boost they need to happen and can create all sorts of new and exciting products.
