Benzenediazonium chloride
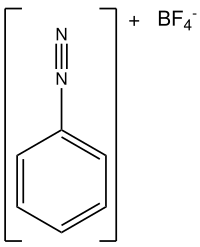
Hey there kiddo! Today we're going to talk about benzenediazonium chloride. It's a big word, but don't worry – I'm going to explain it to you like you're 5 years old.
First of all, let's break down the word. "Benzene" is a type of molecule that's shaped like a hexagon, kind of like a stop sign. "Diazonium" means that there are two nitrogen atoms (the "diaz" part) in the molecule. And "chloride" just means that there's a chlorine atom somewhere in there too.
So, okay, what is benzenediazonium chloride? Well, it's a type of chemical that scientists use to do lots of different things. One of the most common things they use it for is to make other chemicals.
When scientists want to make a new chemical, they often start with a molecule that's already known and tweak it until they get what they want. In the case of benzenediazonium chloride, they start with a molecule called aniline (which is also made up of a benzene ring with an amino group attached to it). Then, they add some chemicals to it to make it turn into benzenediazonium chloride.
Once they have benzenediazonium chloride, they can use it to make all sorts of other things. For example, they might add it to a solution of amines (which are molecules that have nitrogen atoms in them) to make new molecules called diazo dyes, which are used to color fabrics.
Another thing scientists can do with benzenediazonium chloride is to make it react with other molecules to form new compounds. This is called "diazo coupling." By controlling the conditions of the reaction (like how much benzenediazonium chloride they use, or what temperature they do the reaction at), they can get different products with different properties.
So, there you have it – benzenediazonium chloride is a type of chemical that scientists use to make new things. It's made from aniline, has two nitrogen atoms and a chlorine atom in it, and can be used in reactions to form new compounds. Pretty neat, huh?
First of all, let's break down the word. "Benzene" is a type of molecule that's shaped like a hexagon, kind of like a stop sign. "Diazonium" means that there are two nitrogen atoms (the "diaz" part) in the molecule. And "chloride" just means that there's a chlorine atom somewhere in there too.
So, okay, what is benzenediazonium chloride? Well, it's a type of chemical that scientists use to do lots of different things. One of the most common things they use it for is to make other chemicals.
When scientists want to make a new chemical, they often start with a molecule that's already known and tweak it until they get what they want. In the case of benzenediazonium chloride, they start with a molecule called aniline (which is also made up of a benzene ring with an amino group attached to it). Then, they add some chemicals to it to make it turn into benzenediazonium chloride.
Once they have benzenediazonium chloride, they can use it to make all sorts of other things. For example, they might add it to a solution of amines (which are molecules that have nitrogen atoms in them) to make new molecules called diazo dyes, which are used to color fabrics.
Another thing scientists can do with benzenediazonium chloride is to make it react with other molecules to form new compounds. This is called "diazo coupling." By controlling the conditions of the reaction (like how much benzenediazonium chloride they use, or what temperature they do the reaction at), they can get different products with different properties.
So, there you have it – benzenediazonium chloride is a type of chemical that scientists use to make new things. It's made from aniline, has two nitrogen atoms and a chlorine atom in it, and can be used in reactions to form new compounds. Pretty neat, huh?
