Carbonyl group
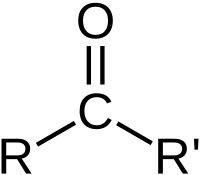
A carbonyl group is a special group of atoms that can be found in molecules. It is made up of two types of atoms: carbon and oxygen. The carbon atom is connected to the oxygen atom with a double bond, which means they share two electrons.
Imagine you have a toy car (carbon atom) and a ball (oxygen atom). If you connect the car and ball with a piece of string (double bond) and pull on each end of the string, the car and ball will stay together. This is what happens with carbon and oxygen in a carbonyl group.
Now, why is this important? Well, a carbonyl group is important because it changes the properties of a molecule. If a molecule has a carbonyl group, it can react with other molecules in different ways. This can affect things like how the molecule smells, how it looks, and how it behaves.
Think of it like adding a hat to a toy. Depending on the type of hat you put on the toy's head, it can change the way the toy looks and the way you interact with it. A carbonyl group does a similar thing to a molecule - it changes the way the molecule behaves and interacts with other molecules.
In summary, a carbonyl group is a special group of atoms (carbon and oxygen) that can be found in molecules. It changes the properties of the molecule, affecting things like smell, appearance, and behavior.
Imagine you have a toy car (carbon atom) and a ball (oxygen atom). If you connect the car and ball with a piece of string (double bond) and pull on each end of the string, the car and ball will stay together. This is what happens with carbon and oxygen in a carbonyl group.
Now, why is this important? Well, a carbonyl group is important because it changes the properties of a molecule. If a molecule has a carbonyl group, it can react with other molecules in different ways. This can affect things like how the molecule smells, how it looks, and how it behaves.
Think of it like adding a hat to a toy. Depending on the type of hat you put on the toy's head, it can change the way the toy looks and the way you interact with it. A carbonyl group does a similar thing to a molecule - it changes the way the molecule behaves and interacts with other molecules.
In summary, a carbonyl group is a special group of atoms (carbon and oxygen) that can be found in molecules. It changes the properties of the molecule, affecting things like smell, appearance, and behavior.
Related topics others have asked about:
