Cations
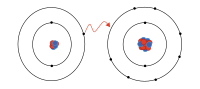
Cations are like happy, friendly little buddies that form when an atom loses one or more of its negatively charged electrons. You know how atoms have a bunch of little electrons spinning around them? Well, sometimes atoms get really excited and lose one or more of those electrons, and when they do, they become positively charged. That positively charged atom is now called a cation, which is like a special club that only certain atoms can be a part of if they lose some of their electrons.
Now, since cations have a positive charge, they are really attracted to things that have a negative charge. It's almost like a magnet, where the positive and negative charges are drawn to each other. That's why cations are always hanging out with anions, which are negatively charged particles.
Cations are really important because they have a big impact on how different things react with each other. If you have a bunch of cations and anions together, they can form really cool things like salts or complexes, which can have all sorts of useful properties. And since cations are so friendly and like to make friends with anions, they can help us figure out how different things will react with each other and what kinds of chemicals we can make.
So that's what cations are all about. They are positively charged atoms that form a special club when they lose some of their electrons, and they are always trying to find some negatively charged anions to hang out with. They are really important for understanding how different chemicals react with each other, so we can make all sorts of cool things!
Now, since cations have a positive charge, they are really attracted to things that have a negative charge. It's almost like a magnet, where the positive and negative charges are drawn to each other. That's why cations are always hanging out with anions, which are negatively charged particles.
Cations are really important because they have a big impact on how different things react with each other. If you have a bunch of cations and anions together, they can form really cool things like salts or complexes, which can have all sorts of useful properties. And since cations are so friendly and like to make friends with anions, they can help us figure out how different things will react with each other and what kinds of chemicals we can make.
So that's what cations are all about. They are positively charged atoms that form a special club when they lose some of their electrons, and they are always trying to find some negatively charged anions to hang out with. They are really important for understanding how different chemicals react with each other, so we can make all sorts of cool things!
Related topics others have asked about:
