Fluorine-19 nuclear magnetic resonance spectroscopy
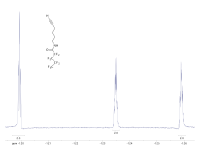
Hey kiddo, today we're going to talk about fluorine-19 nuclear magnetic resonance spectroscopy. Sounds like a mouthful, doesn't it?
Okay, so let's break it down. Molecules are made up of atoms, and those atoms have a nucleus that spins around. We can think of it like a tiny top or spinning toy.
Now, inside that spinning nucleus, there are positively charged particles called protons and negatively charged particles called electrons.
When we put a molecule in a magnetic field and zap it with radio waves, the spinning nucleus gets excited and starts wobbling around like a toy top that's been spun.
This wobbling creates a tiny magnetic field, which we can measure with a special machine called a spectrometer.
Fluorine-19 is a type of atom that's really good for this kind of measurement. By measuring the wobbling of fluorine-19, we can learn a lot about the molecule it's in.
Think of it like a fingerprint for molecules. Each molecule has a unique set of wobbles, just like every person has a unique set of fingerprints.
Scientists use this information to study all kinds of things, from drugs to plastics to proteins. It's like looking inside a molecule to see what's going on in there.
So that's fluorine-19 nuclear magnetic resonance spectroscopy! A fancy way of looking inside molecules and learning about their unique wobbles.
Okay, so let's break it down. Molecules are made up of atoms, and those atoms have a nucleus that spins around. We can think of it like a tiny top or spinning toy.
Now, inside that spinning nucleus, there are positively charged particles called protons and negatively charged particles called electrons.
When we put a molecule in a magnetic field and zap it with radio waves, the spinning nucleus gets excited and starts wobbling around like a toy top that's been spun.
This wobbling creates a tiny magnetic field, which we can measure with a special machine called a spectrometer.
Fluorine-19 is a type of atom that's really good for this kind of measurement. By measuring the wobbling of fluorine-19, we can learn a lot about the molecule it's in.
Think of it like a fingerprint for molecules. Each molecule has a unique set of wobbles, just like every person has a unique set of fingerprints.
Scientists use this information to study all kinds of things, from drugs to plastics to proteins. It's like looking inside a molecule to see what's going on in there.
So that's fluorine-19 nuclear magnetic resonance spectroscopy! A fancy way of looking inside molecules and learning about their unique wobbles.
