Hydrogenation of carbon–nitrogen double bonds
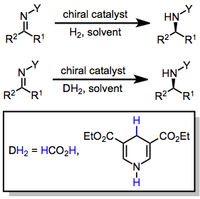
Okay kiddo, have you heard of something called the carbon–nitrogen double bond? It's like a special connection between a carbon and nitrogen atom in a molecule. Now sometimes we want to change this connection to make the molecule more useful or have different properties. And one way we can do this is through something called hydrogenation.
Hydrogenation is like adding hydrogen atoms to the molecule. It's like getting more friends to join the party! We use something called a catalyst, which is like a helper molecule, to make this happen.
When we add hydrogen atoms to the carbon–nitrogen double bond, it changes the connection between the atoms. It's like turning a key to unlock a door, making it easier for things to come in and out. This change in connection is called a reduction reaction.
Reducing the carbon-nitrogen double bond can change how the molecule behaves, making it more or less reactive. This can be useful for things like making medicines, plastics, or food ingredients.
So, the hydrogenation of carbon–nitrogen double bonds is basically adding hydrogen atoms to a special connection in a molecule to change its properties. It's like getting more friends to join the party and changing how the molecule behaves.
Hydrogenation is like adding hydrogen atoms to the molecule. It's like getting more friends to join the party! We use something called a catalyst, which is like a helper molecule, to make this happen.
When we add hydrogen atoms to the carbon–nitrogen double bond, it changes the connection between the atoms. It's like turning a key to unlock a door, making it easier for things to come in and out. This change in connection is called a reduction reaction.
Reducing the carbon-nitrogen double bond can change how the molecule behaves, making it more or less reactive. This can be useful for things like making medicines, plastics, or food ingredients.
So, the hydrogenation of carbon–nitrogen double bonds is basically adding hydrogen atoms to a special connection in a molecule to change its properties. It's like getting more friends to join the party and changing how the molecule behaves.
