Jablonski diagram
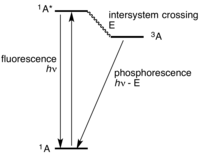
Okay kiddo, a Jablonski diagram is a special diagram that helps scientists understand what happens when light shines on molecules or atoms.
Imagine you have a toy car that runs on a battery. When you turn on the switch, the battery gives power to the car and it starts moving. In the same way, when light shines on a molecule, it gives energy to the molecule and changes how it behaves.
The Jablonski diagram shows all the different things that can happen to a molecule when it gets energy from light. On the diagram, there is a line that represents the different energy levels a molecule can have. Think of it like a ladder, with each rung representing a different energy level.
When light shines on a molecule, it can jump up to a higher energy level on the ladder. This is called "excitation". The molecule is now "excited" and has more energy than before.
After it's excited, the molecule can either jump back down to its original energy level (this is called fluorescence or phosphorescence), or it can release the extra energy it has as heat. Some molecules can also transfer their energy to other nearby molecules, which can cause them to become excited too.
So the Jablonski diagram helps scientists understand the different ways that molecules can react to light. Just like how you use a map to understand the different roads and destinations, scientists use the Jablonski diagram to understand how molecules react to light. Cool, huh?
Imagine you have a toy car that runs on a battery. When you turn on the switch, the battery gives power to the car and it starts moving. In the same way, when light shines on a molecule, it gives energy to the molecule and changes how it behaves.
The Jablonski diagram shows all the different things that can happen to a molecule when it gets energy from light. On the diagram, there is a line that represents the different energy levels a molecule can have. Think of it like a ladder, with each rung representing a different energy level.
When light shines on a molecule, it can jump up to a higher energy level on the ladder. This is called "excitation". The molecule is now "excited" and has more energy than before.
After it's excited, the molecule can either jump back down to its original energy level (this is called fluorescence or phosphorescence), or it can release the extra energy it has as heat. Some molecules can also transfer their energy to other nearby molecules, which can cause them to become excited too.
So the Jablonski diagram helps scientists understand the different ways that molecules can react to light. Just like how you use a map to understand the different roads and destinations, scientists use the Jablonski diagram to understand how molecules react to light. Cool, huh?
Related topics others have asked about:
