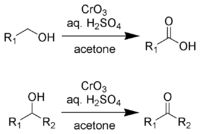
Jones oxidation
Okay, kiddo, have you ever seen an apple turn brown when it's cut open and left out for too long? That's because of something called oxidation.
Well, in chemistry, there is a reaction called Jones oxidation that also involves oxidation. It's a way to turn certain types of molecules into different types by adding oxygen atoms to them.
Let's say we start with a molecule that has a double bond between two carbon atoms. That means there are two carbons next to each other that are sharing some electrons. We can use Jones oxidation to turn that double bond into two separate carbon-oxygen bonds, which makes a completely different molecule.
To do this, we need three things: the molecule we want to change, some acid (like sulfuric acid), and a type of chemical called a chromate or a dichromate. These chemicals have lots of oxygen atoms in them which can be given to the molecule we want to change.
We mix all of these things together and wait for a little while. During that time, the chromate or dichromate gives its oxygen atoms to the double bond in the molecule we want to change. This makes it break apart and form new carbon-oxygen bonds instead.
Once this reaction is finished, we have a new molecule with different properties than it had before. And all because we added a bunch of oxygen atoms to it with Jones oxidation!
Well, in chemistry, there is a reaction called Jones oxidation that also involves oxidation. It's a way to turn certain types of molecules into different types by adding oxygen atoms to them.
Let's say we start with a molecule that has a double bond between two carbon atoms. That means there are two carbons next to each other that are sharing some electrons. We can use Jones oxidation to turn that double bond into two separate carbon-oxygen bonds, which makes a completely different molecule.
To do this, we need three things: the molecule we want to change, some acid (like sulfuric acid), and a type of chemical called a chromate or a dichromate. These chemicals have lots of oxygen atoms in them which can be given to the molecule we want to change.
We mix all of these things together and wait for a little while. During that time, the chromate or dichromate gives its oxygen atoms to the double bond in the molecule we want to change. This makes it break apart and form new carbon-oxygen bonds instead.
Once this reaction is finished, we have a new molecule with different properties than it had before. And all because we added a bunch of oxygen atoms to it with Jones oxidation!
