Limiting reagent
Imagine you are baking cookies and you need a cup of sugar and a cup of flour for your recipe. But you only have half a cup of sugar and a whole cup of flour. You can't make the cookies with just flour, so you have to use the amount of sugar you have as the limit for the recipe. That half cup of sugar is the limiting reagent.
In science, when we mix different chemicals together, some of them may react and turn into something else. The reactants are the things we mix, and the products are the final result of the reaction.
But sometimes we have too much of one reactant, and not enough of another. Just like in the cookie example, the amount of one reactant can limit the amount of product that we can make. This is called the limiting reagent.
The limiting reagent is the reactant that gets used up first, and it limits how much product can be formed. It's like the half cup of sugar in the cookie example. If we run out of the limiting reagent, the reaction will stop, and no more product will be made.
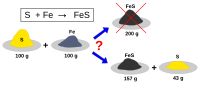
In chemistry, we use stoichiometry to figure out which reactant is the limiting reagent. We compare the amount of each reactant we have to the balanced equation for the reaction. The reactant that produces the least amount of product is the limiting reagent.
So, just like with baking cookies, the limiting reagent is the thing that limits how much product we can make when we mix chemicals together.
In science, when we mix different chemicals together, some of them may react and turn into something else. The reactants are the things we mix, and the products are the final result of the reaction.
But sometimes we have too much of one reactant, and not enough of another. Just like in the cookie example, the amount of one reactant can limit the amount of product that we can make. This is called the limiting reagent.
The limiting reagent is the reactant that gets used up first, and it limits how much product can be formed. It's like the half cup of sugar in the cookie example. If we run out of the limiting reagent, the reaction will stop, and no more product will be made.
In chemistry, we use stoichiometry to figure out which reactant is the limiting reagent. We compare the amount of each reactant we have to the balanced equation for the reaction. The reactant that produces the least amount of product is the limiting reagent.
So, just like with baking cookies, the limiting reagent is the thing that limits how much product we can make when we mix chemicals together.
Related topics others have asked about:
