Multipole density formalism
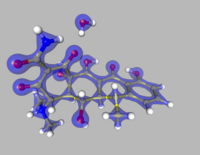
Multipole density formalism is a way to describe the distribution of electric charge in a molecule, especially for large molecules. Think of atoms bonded together to form a molecule - this formalism helps to explain how the electrons orbiting around each nucleus interact with each other to create a net charge distribution.
Now, imagine a toy set of blocks with different shapes (spheres, cones, cylinders, and more) stacked on top of each other to make a tower. Each block represents an atom in a molecule.
In the multipole density formalism, we add together mathematical terms for each block that describe how much positive or negative charge is present and how it is distributed in three-dimensional space. These terms are calculated based on the shape and position of each block in the tower.
Then, we add up all of the terms to get a final mathematical expression that describes the net charge distribution of the whole molecule. This information helps us understand how the molecule interacts with other molecules and how it affects chemical reactions.
So, the multipole density formalism is like a way to take a toy set of blocks and use math to describe and analyze the electrical charge in a molecule!
Now, imagine a toy set of blocks with different shapes (spheres, cones, cylinders, and more) stacked on top of each other to make a tower. Each block represents an atom in a molecule.
In the multipole density formalism, we add together mathematical terms for each block that describe how much positive or negative charge is present and how it is distributed in three-dimensional space. These terms are calculated based on the shape and position of each block in the tower.
Then, we add up all of the terms to get a final mathematical expression that describes the net charge distribution of the whole molecule. This information helps us understand how the molecule interacts with other molecules and how it affects chemical reactions.
So, the multipole density formalism is like a way to take a toy set of blocks and use math to describe and analyze the electrical charge in a molecule!
