Pressure solution
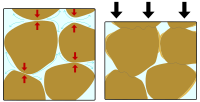
Pressure solution is when solid materials dissolve or break down under pressure. Let's imagine you have a piece of candy. When you put it in your mouth and chew it, it gets smaller and smaller, right? That's because your teeth are putting pressure on the candy, and it begins to break apart.
In a similar way, pressure solution happens in nature when rocks are placed under a lot of pressure. Rocks are made up of tiny particles called minerals, which are held together by chemical bonds. When the rocks are squeezed together, like when you squeeze a ball of clay with your hands, the pressure forces the minerals to start dissolving and breaking apart.
Now, when the minerals start dissolving, they release tiny particles called ions, which are like little building blocks of the mineral. These ions can then move and spread out in the water or any other fluids present, like when you dissolve sugar in water to make a sweet solution.
But how does this happen? Well, the pressure pushes the minerals against each other, making their atoms really close. The atoms at the surface of each mineral start interacting with the atoms of the neighboring mineral. This interaction causes the bonds between the atoms of the minerals to break.
Once the bonds break, the minerals start to dissolve, just like putting a sugar cube in water and watching it disappear. The dissolved ions then move away from the mineral and mix with the water or fluid around it.
Pressure solution can happen over a long period of time, and it happens more easily when there is some heat involved. That's why pressure solution is often observed deep underground, where there are high temperatures and lots of pressure.
By understanding how pressure solution works, geologists can learn about the history of rocks and the forces that acted upon them. This knowledge is essential in understanding how the Earth's surface has changed over millions of years.
In a similar way, pressure solution happens in nature when rocks are placed under a lot of pressure. Rocks are made up of tiny particles called minerals, which are held together by chemical bonds. When the rocks are squeezed together, like when you squeeze a ball of clay with your hands, the pressure forces the minerals to start dissolving and breaking apart.
Now, when the minerals start dissolving, they release tiny particles called ions, which are like little building blocks of the mineral. These ions can then move and spread out in the water or any other fluids present, like when you dissolve sugar in water to make a sweet solution.
But how does this happen? Well, the pressure pushes the minerals against each other, making their atoms really close. The atoms at the surface of each mineral start interacting with the atoms of the neighboring mineral. This interaction causes the bonds between the atoms of the minerals to break.
Once the bonds break, the minerals start to dissolve, just like putting a sugar cube in water and watching it disappear. The dissolved ions then move away from the mineral and mix with the water or fluid around it.
Pressure solution can happen over a long period of time, and it happens more easily when there is some heat involved. That's why pressure solution is often observed deep underground, where there are high temperatures and lots of pressure.
By understanding how pressure solution works, geologists can learn about the history of rocks and the forces that acted upon them. This knowledge is essential in understanding how the Earth's surface has changed over millions of years.
Related topics others have asked about:
