Quaternary ammonium cation
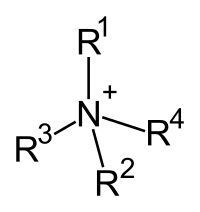
A quaternary ammonium cation is a special thing called a molecule. A molecule is like a tiny Lego block made up of atoms. Quaternary ammonium cations are made up of four special things called nitrogen and one other thing called a smaller molecule that likes to attach itself to nitrogen called an alkyl or aryl group.
This molecule is special because it has a positive charge. We call it a cation because of this charge. Think of it like when you put two magnets together and they stick, one side has a positive charge and one side has a negative charge. The positive charge on the quaternary ammonium cation makes it stick to other things that have a negative charge, like a magnet.
We use quaternary ammonium cations to kill bad germs on things like our hands, floors, and countertops. They do this by sticking to those bad germs and messing up their special outer layer, like when you take apart a Lego tower. The germs can’t survive without this special layer and die.
But even though quaternary ammonium cations are awesome at killing germs, we need to be careful with them because they can also hurt the good germs we need on our skin and surfaces to stay healthy. That’s why when we use things with quaternary ammonium cations, we follow the instructions carefully so we don’t hurt ourselves or the good germs!
This molecule is special because it has a positive charge. We call it a cation because of this charge. Think of it like when you put two magnets together and they stick, one side has a positive charge and one side has a negative charge. The positive charge on the quaternary ammonium cation makes it stick to other things that have a negative charge, like a magnet.
We use quaternary ammonium cations to kill bad germs on things like our hands, floors, and countertops. They do this by sticking to those bad germs and messing up their special outer layer, like when you take apart a Lego tower. The germs can’t survive without this special layer and die.
But even though quaternary ammonium cations are awesome at killing germs, we need to be careful with them because they can also hurt the good germs we need on our skin and surfaces to stay healthy. That’s why when we use things with quaternary ammonium cations, we follow the instructions carefully so we don’t hurt ourselves or the good germs!
Related topics others have asked about:
