Sodium–sulfur battery
Okay kiddo, imagine a toy box with two different toys, one is a yellow ball (sodium) and the other is a green cube (sulfur). Now, let's imagine we want to save some of the energy these toys have so that we can use it later on when we are playing with them.
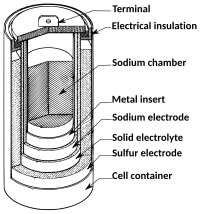
The sodium-sulfur battery works in a similar way. It has two parts, one is called the anode (where the sodium lives) and the other is called the cathode (where the sulfur lives). When we want to save energy, we put the sodium and sulfur together in the anode and cathode cells. This creates a reaction that produces electricity, and we can store this electricity until we need it later on.
This type of battery is really good for things that need lots of energy like cars, buses, and even cities! It's also very efficient because when we want to use the stored energy, we just reverse the reaction and the sodium and sulfur separate again, which creates electricity. So we get to use the energy all over again, without wasting any of it!
That's it! Pretty cool, huh?
The sodium-sulfur battery works in a similar way. It has two parts, one is called the anode (where the sodium lives) and the other is called the cathode (where the sulfur lives). When we want to save energy, we put the sodium and sulfur together in the anode and cathode cells. This creates a reaction that produces electricity, and we can store this electricity until we need it later on.
This type of battery is really good for things that need lots of energy like cars, buses, and even cities! It's also very efficient because when we want to use the stored energy, we just reverse the reaction and the sodium and sulfur separate again, which creates electricity. So we get to use the energy all over again, without wasting any of it!
That's it! Pretty cool, huh?
Related topics others have asked about:
