Transition metal complexes of aldehydes and ketones
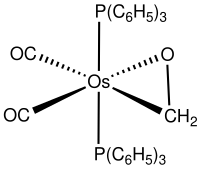
Okay, so let's start with what are transition metals. Transition metals are a group of special metals that have some unique properties. They are called transition metals because their electrons move around a lot, or "transition," between different energy levels. These metals include things like iron, copper, and gold.
Now, let's talk about aldehydes and ketones. Aldehydes and ketones are special types of compounds called organic compounds. They are made up of carbon, hydrogen, and oxygen atoms. They have a special group of atoms called a carbonyl group. In aldehydes, the carbonyl group is at the end of the carbon chain, and in ketones, it is in the middle.
Now, when you combine transition metals and aldehydes or ketones, something interesting happens. The transition metal can form a complex with the aldehyde or ketone. A complex is like when two things join together to make something new.
In this case, the transition metal forms bonds with the carbon and oxygen in the carbonyl group. These bonds help to stabilize the aldehyde or ketone and make it more stable. This is important because aldehydes and ketones can be very reactive and easily change into different compounds.
The transition metal also changes its properties when it forms a complex with the aldehyde or ketone. It can become more reactive or have different colors or magnetic properties.
This formation of a complex between the transition metal and the aldehyde or ketone is so important in many chemical reactions. It helps to speed up reactions or make them happen in certain ways.
So, in summary, transition metal complexes of aldehydes and ketones are special combinations of transition metals and organic compounds that help stabilize the compounds and change the properties of the metals. They are important in many chemical reactions.
Now, let's talk about aldehydes and ketones. Aldehydes and ketones are special types of compounds called organic compounds. They are made up of carbon, hydrogen, and oxygen atoms. They have a special group of atoms called a carbonyl group. In aldehydes, the carbonyl group is at the end of the carbon chain, and in ketones, it is in the middle.
Now, when you combine transition metals and aldehydes or ketones, something interesting happens. The transition metal can form a complex with the aldehyde or ketone. A complex is like when two things join together to make something new.
In this case, the transition metal forms bonds with the carbon and oxygen in the carbonyl group. These bonds help to stabilize the aldehyde or ketone and make it more stable. This is important because aldehydes and ketones can be very reactive and easily change into different compounds.
The transition metal also changes its properties when it forms a complex with the aldehyde or ketone. It can become more reactive or have different colors or magnetic properties.
This formation of a complex between the transition metal and the aldehyde or ketone is so important in many chemical reactions. It helps to speed up reactions or make them happen in certain ways.
So, in summary, transition metal complexes of aldehydes and ketones are special combinations of transition metals and organic compounds that help stabilize the compounds and change the properties of the metals. They are important in many chemical reactions.
