Tris(2,3-dibromopropyl) phosphate
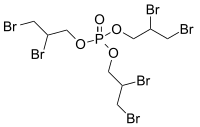
Tris(2,3-dibromopropyl) phosphate is like a super special juice box for fire. You know how you have a fire extinguisher in your home to put out fires? This juice box works just like that but is used by firefighters to put out BIG fires!
But how does it work? Well, let's break it down.
First, you know how water puts out fires by cooling them down? This juice box works differently. It contains chemicals that stop the fire from continuing to burn. These chemicals take away the fire's food and oxygen which makes it stop burning.
Now, the juice box is made up of three parts called "tris", "dibromopropyl" and "phosphate". Think of these as three different colors of Legos. You stack them together in a certain way to create something new and special.
Tris is a group of three special parts that are really good at sticking to other things. Dibromopropyl is a group of special parts that contain bromine, which is really good at reacting with fire. Put them together and you have three bromine parts that are "sticking" to tris.
Finally, phosphate is a special part that keeps everything in the juice box together. It's like the glue that holds everything in place.
So when firefighters use tris(2,3-dibromopropyl) phosphate, they release the juice onto the fire. When the juice lands on the fire, the bromine parts react with it and take away its ability to burn, putting the fire out!
Just remember that even though the juice box can put out fires, it's still really important to be careful with fire and not play with it!
But how does it work? Well, let's break it down.
First, you know how water puts out fires by cooling them down? This juice box works differently. It contains chemicals that stop the fire from continuing to burn. These chemicals take away the fire's food and oxygen which makes it stop burning.
Now, the juice box is made up of three parts called "tris", "dibromopropyl" and "phosphate". Think of these as three different colors of Legos. You stack them together in a certain way to create something new and special.
Tris is a group of three special parts that are really good at sticking to other things. Dibromopropyl is a group of special parts that contain bromine, which is really good at reacting with fire. Put them together and you have three bromine parts that are "sticking" to tris.
Finally, phosphate is a special part that keeps everything in the juice box together. It's like the glue that holds everything in place.
So when firefighters use tris(2,3-dibromopropyl) phosphate, they release the juice onto the fire. When the juice lands on the fire, the bromine parts react with it and take away its ability to burn, putting the fire out!
Just remember that even though the juice box can put out fires, it's still really important to be careful with fire and not play with it!
Related topics others have asked about:
