Valence electrons
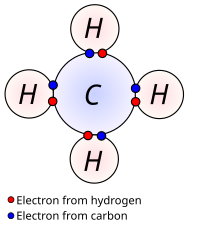
Okay kiddo, let's talk about valence electrons. First, let's think about an atom as a little house with rooms inside. Inside each room, there are little particles called electrons that flies around in circles. Some electrons are in the basement, some are in the first floor and some are upstairs.
Now, valence electrons are the ones that are in the outermost room (called the "valence shell") of the house or the outermost circle of the atom. These electrons are super important because they determine how the atom interacts with other atoms.
You see, atoms like to be happy and they do that by filling up their rooms with electrons. The valence electrons are the ones that can be shared, given away or borrowed by other atoms. When atoms share electrons, it's called a covalent bond. When one atom gives an electron to another, that's called an ionic bond.
Valence electrons also determine what kind of chemical reactions an atom can undergo. For example, if an atom has a lot of valence electrons, it's less likely to give them away because it's already pretty happy. But if an atom doesn't have many valence electrons, it may be more likely to give them away so it can be happy too.
So you see, valence electrons are like the little kids in the outermost room of an atom's house. They hold the keys to the atom's happiness and relationships with other atoms.
Now, valence electrons are the ones that are in the outermost room (called the "valence shell") of the house or the outermost circle of the atom. These electrons are super important because they determine how the atom interacts with other atoms.
You see, atoms like to be happy and they do that by filling up their rooms with electrons. The valence electrons are the ones that can be shared, given away or borrowed by other atoms. When atoms share electrons, it's called a covalent bond. When one atom gives an electron to another, that's called an ionic bond.
Valence electrons also determine what kind of chemical reactions an atom can undergo. For example, if an atom has a lot of valence electrons, it's less likely to give them away because it's already pretty happy. But if an atom doesn't have many valence electrons, it may be more likely to give them away so it can be happy too.
So you see, valence electrons are like the little kids in the outermost room of an atom's house. They hold the keys to the atom's happiness and relationships with other atoms.
