Valence shell
Imagine you are playing with blocks of different shapes and sizes. You have a big square block and a small triangular block. The big square block has four sides and can connect with four other blocks at the same time. The small triangular block, on the other hand, only has three sides and can only connect with three other blocks at the same time.
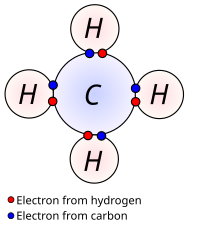
In chemistry, atoms are like the blocks, and they have a special part called the valence shell. The valence shell is like the number of sides of the block, and it shows how many other atoms an atom can connect with. Just like how the big square block has more sides than the small triangular block, some atoms have more valence electrons (the electrons in the valence shell) than others.
Atoms really want to fill up their valence shell with electrons. It's like they want to connect with as many other atoms as they can to make a bigger and stronger block structure. When atoms are able to share electrons with other atoms or even borrow electrons from other atoms, they form chemical bonds. These bonds help atoms to fill up their valence shells and form more stable structures.
So basically, the valence shell is a special part of an atom that shows how many other atoms it can connect with. And when atoms are able to fill up their valence shells, they become more stable and form chemical bonds with other atoms.
In chemistry, atoms are like the blocks, and they have a special part called the valence shell. The valence shell is like the number of sides of the block, and it shows how many other atoms an atom can connect with. Just like how the big square block has more sides than the small triangular block, some atoms have more valence electrons (the electrons in the valence shell) than others.
Atoms really want to fill up their valence shell with electrons. It's like they want to connect with as many other atoms as they can to make a bigger and stronger block structure. When atoms are able to share electrons with other atoms or even borrow electrons from other atoms, they form chemical bonds. These bonds help atoms to fill up their valence shells and form more stable structures.
So basically, the valence shell is a special part of an atom that shows how many other atoms it can connect with. And when atoms are able to fill up their valence shells, they become more stable and form chemical bonds with other atoms.
