Anions
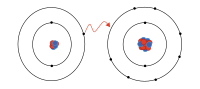
Anions are like the grumpy part of a family that doesn't want to share their toys with others. They are particles that have a negative charge, which means they have more electrons than protons in their atoms.
Now, you might be wondering, what are electrons and protons? Electrons are tiny, spinning particles that orbit around the center of an atom. They have a negative charge, and the more electrons an atom has, the more negative it becomes. Protons are also tiny particles inside an atom, but they have a positive charge. When there are equal numbers of electrons and protons in an atom, the atom doesn't have a charge – it's neutral.
However, sometimes atoms lose or gain electrons, which changes their charge. When an atom loses an electron, it becomes positive because there are now more protons than electrons. When it gains an electron, it becomes negative because there are now more electrons than protons. These negatively charged atoms are called anions.
Now, why would an atom gain or lose electrons? Well, it all depends on the situation. Sometimes an atom might lose an electron because it's trying to bond with another atom that wants to gain an electron. By losing an electron, the first atom becomes positive and the second atom becomes negative, and they are attracted to each other. This is how molecules are formed. Other times, an atom might gain an electron to fill up its outermost shell and become more stable.
Overall, anions might sound complicated, but they're just atoms that have gained extra electrons and become negative. Think of them like grumpy family members who want to keep all their toys to themselves!
Now, you might be wondering, what are electrons and protons? Electrons are tiny, spinning particles that orbit around the center of an atom. They have a negative charge, and the more electrons an atom has, the more negative it becomes. Protons are also tiny particles inside an atom, but they have a positive charge. When there are equal numbers of electrons and protons in an atom, the atom doesn't have a charge – it's neutral.
However, sometimes atoms lose or gain electrons, which changes their charge. When an atom loses an electron, it becomes positive because there are now more protons than electrons. When it gains an electron, it becomes negative because there are now more electrons than protons. These negatively charged atoms are called anions.
Now, why would an atom gain or lose electrons? Well, it all depends on the situation. Sometimes an atom might lose an electron because it's trying to bond with another atom that wants to gain an electron. By losing an electron, the first atom becomes positive and the second atom becomes negative, and they are attracted to each other. This is how molecules are formed. Other times, an atom might gain an electron to fill up its outermost shell and become more stable.
Overall, anions might sound complicated, but they're just atoms that have gained extra electrons and become negative. Think of them like grumpy family members who want to keep all their toys to themselves!
Related topics others have asked about:
