Atomic electron transition
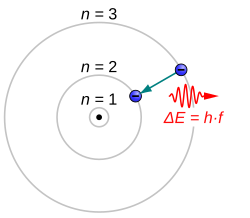
Atoms are like little balls with tiny little things called electrons buzzing around them. These electrons are really important because they determine how the atom behaves and what it is like. Sometimes, an electron in an atom gets excited and jumps from one energy level to another. This is called an atomic electron transition!
Think of it like when you get really excited and jump up and down. You have a lot of energy and want to release it somehow. That's kind of what the electron is feeling when it moves between energy levels.
So, what are energy levels? They are like steps on a staircase, but instead of going up and down, electrons hop between them. Each energy level has a different amount of energy, so if an electron moves to a higher energy level, it gets more excited and has more energy.
When an electron transitions from a higher energy level to a lower energy level, it releases energy in the form of light. This is kind of like when you jump off the couch and land on the ground, you make a 'thump' sound. But instead of a sound, the electron is releasing light. This is why things like neon lights or fireworks look so colorful!
So, atomic electron transition is when an electron in an atom gets excited, moves to a different energy level, and then releases energy in the form of light.
Think of it like when you get really excited and jump up and down. You have a lot of energy and want to release it somehow. That's kind of what the electron is feeling when it moves between energy levels.
So, what are energy levels? They are like steps on a staircase, but instead of going up and down, electrons hop between them. Each energy level has a different amount of energy, so if an electron moves to a higher energy level, it gets more excited and has more energy.
When an electron transitions from a higher energy level to a lower energy level, it releases energy in the form of light. This is kind of like when you jump off the couch and land on the ground, you make a 'thump' sound. But instead of a sound, the electron is releasing light. This is why things like neon lights or fireworks look so colorful!
So, atomic electron transition is when an electron in an atom gets excited, moves to a different energy level, and then releases energy in the form of light.
Related topics others have asked about:
