Bohr atom
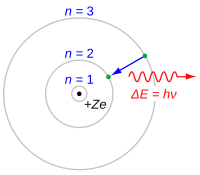
Have you ever seen a picture of an atom? It's kind of like a tiny little universe where there are really tiny things called electrons that go around a bigger thing called the nucleus.
One really smart guy named Niels Bohr thought a lot about atoms and how they work. He figured out that electrons don't just go around the nucleus in any old way, but they have specific places they like to be called energy levels. It's kind of like how people have favorite spots to sit at the dinner table.
Each energy level has a certain amount of energy that goes with it, and each electron has to have just the right amount of energy to be in that level. It's kind of like how a balloon has to have just the right amount of air to float in the air.
Bohr also figured out that when an electron gets just the right amount of energy, it can jump up to a higher energy level. But it can't stay there forever, so it eventually jumps back down and gives off a little bit of extra energy in the form of light or heat. It's kind of like when you jump really high and then come back down, you might make a little noise or feel a little hot because of the energy you used.
So that's the Bohr Atom! It's a tiny little universe where electrons have their own energy levels, and they can jump up and down and give off energy.
One really smart guy named Niels Bohr thought a lot about atoms and how they work. He figured out that electrons don't just go around the nucleus in any old way, but they have specific places they like to be called energy levels. It's kind of like how people have favorite spots to sit at the dinner table.
Each energy level has a certain amount of energy that goes with it, and each electron has to have just the right amount of energy to be in that level. It's kind of like how a balloon has to have just the right amount of air to float in the air.
Bohr also figured out that when an electron gets just the right amount of energy, it can jump up to a higher energy level. But it can't stay there forever, so it eventually jumps back down and gives off a little bit of extra energy in the form of light or heat. It's kind of like when you jump really high and then come back down, you might make a little noise or feel a little hot because of the energy you used.
So that's the Bohr Atom! It's a tiny little universe where electrons have their own energy levels, and they can jump up and down and give off energy.
Related topics others have asked about:
