Bohr model
So you know how we have an idea in our head of what an atom looks like, kind of like a bunch of tiny balls or circles stuck together? Well, a long time ago, a very smart man named Niels Bohr came up with a way to explain how atoms work by using a picture called the Bohr model.
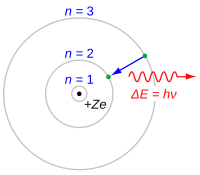
In the Bohr model, there is a center part called the nucleus, which is made up of tiny particles called protons and neutrons. Around the nucleus, there are little bits called electrons that are always moving and orbiting around it like planets around the sun.
Bohr figured out that there are certain specific places, or levels, where an electron can orbit around the nucleus. These levels are kind of like steps on a staircase, where each step is a little bit higher than the one before it. Electrons can't just orbit anywhere - they have to be on one of these levels.
The cool thing is that when an electron moves from one level to another, it gives off light. You know how when you turn on a flashlight, it makes light? It's kind of like that, but with tiny electrons inside an atom. This is called an emission spectrum.
The Bohr model helped scientists understand how atoms work and how electrons move around in them. It's still used today as a starting point for learning about atoms and their behavior!
In the Bohr model, there is a center part called the nucleus, which is made up of tiny particles called protons and neutrons. Around the nucleus, there are little bits called electrons that are always moving and orbiting around it like planets around the sun.
Bohr figured out that there are certain specific places, or levels, where an electron can orbit around the nucleus. These levels are kind of like steps on a staircase, where each step is a little bit higher than the one before it. Electrons can't just orbit anywhere - they have to be on one of these levels.
The cool thing is that when an electron moves from one level to another, it gives off light. You know how when you turn on a flashlight, it makes light? It's kind of like that, but with tiny electrons inside an atom. This is called an emission spectrum.
The Bohr model helped scientists understand how atoms work and how electrons move around in them. It's still used today as a starting point for learning about atoms and their behavior!
Related topics others have asked about:
