Collision theory
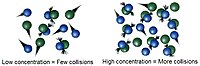
Have you ever played a game of marbles or seen two cars crashing into each other? That's kind of what we talk about when we say "collision theory". It's about how particles, like marbles or atoms, bump into each other and change things.
Imagine you are playing with your marbles and you want to hit one with another to make it move. Well, you have to roll one marble into the other with enough force to make it move. The same idea applies in the world of chemistry. In order to make a chemical reaction happen, the atoms and molecules have to bump into each other with enough force and in the right way.
But wait - why don't the atoms just bounce off each other when they hit? That's where the idea of activation energy comes in. Think of it like a barrier - if the particles have enough energy to get over that barrier, they'll react. If not, they'll just bounce off each other and nothing will happen.
There are a few things that can affect whether or not two particles will react when they collide. First, they have to be going in the right direction - like rolling that marble straight into the other one. Second, they have to have the right amount of energy so they can get past that barrier. Finally, the particles have to be the right type - kind of like how you can't fit a square marble into a round hole.
So next time you're playing with marbles or watching two cars crash (safely, of course), remember that you're seeing a bit of collision theory in action!
Imagine you are playing with your marbles and you want to hit one with another to make it move. Well, you have to roll one marble into the other with enough force to make it move. The same idea applies in the world of chemistry. In order to make a chemical reaction happen, the atoms and molecules have to bump into each other with enough force and in the right way.
But wait - why don't the atoms just bounce off each other when they hit? That's where the idea of activation energy comes in. Think of it like a barrier - if the particles have enough energy to get over that barrier, they'll react. If not, they'll just bounce off each other and nothing will happen.
There are a few things that can affect whether or not two particles will react when they collide. First, they have to be going in the right direction - like rolling that marble straight into the other one. Second, they have to have the right amount of energy so they can get past that barrier. Finally, the particles have to be the right type - kind of like how you can't fit a square marble into a round hole.
So next time you're playing with marbles or watching two cars crash (safely, of course), remember that you're seeing a bit of collision theory in action!
Related topics others have asked about:
