Diastereomer
Okay kiddo, imagine you have a toy box with a bunch of different shaped blocks inside. Now imagine you have two blocks that look almost the same, but when you try to fit them together, they just don't match up right. Those two blocks are called diastereomers!
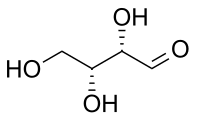
In the world of chemistry, molecules can also have shapes that are almost the same, but not quite. These molecules are called stereoisomers. Diastereomers are a specific type of stereoisomer that have at least two different chiral centers (kinda like having two different shaped blocks on the same molecule).
Now, remember that chiral centers are like your hands - they look almost the same, but they are actually mirror images that can't be superimposed on top of each other. Diastereomers have at least two chiral centers that are different from each other. This makes them unique from other stereoisomers, like enantiomers, which have identical chiral centers.
So, the bottom line here kiddo is that diastereomers are kind of like two different shaped blocks that look similar, but don't fit together. They are a special type of molecule with at least two different chiral centers, making them unique from other types of stereoisomers.
In the world of chemistry, molecules can also have shapes that are almost the same, but not quite. These molecules are called stereoisomers. Diastereomers are a specific type of stereoisomer that have at least two different chiral centers (kinda like having two different shaped blocks on the same molecule).
Now, remember that chiral centers are like your hands - they look almost the same, but they are actually mirror images that can't be superimposed on top of each other. Diastereomers have at least two chiral centers that are different from each other. This makes them unique from other stereoisomers, like enantiomers, which have identical chiral centers.
So, the bottom line here kiddo is that diastereomers are kind of like two different shaped blocks that look similar, but don't fit together. They are a special type of molecule with at least two different chiral centers, making them unique from other types of stereoisomers.
Related topics others have asked about:
