Diphosphane
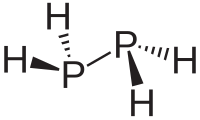
Diphosphane is a chemical that is made up of two phosphorus atoms and four hydrogen atoms. It is also known by its chemical formula, P2H4.
Now, imagine you want to build a tower out of blocks. You can stack one block on top of another block, and keep going until your tower is as tall as you want it to be.
In the same way, atoms can also bond together to form molecules. In the case of diphosphane, the two phosphorus atoms are bonding together to form one molecule, just like stacking blocks together to make a tower.
But wait, there's more! Diphosphane is actually a gas at room temperature and pressure. This means that if you were to try to pick it up with your hands, you wouldn't be able to because it would just float away.
So, to summarize: diphosphane is a gas made up of two phosphorus atoms and four hydrogen atoms that are bonded together to form a molecule. It's like building a tower out of blocks, but with atoms instead.
Now, imagine you want to build a tower out of blocks. You can stack one block on top of another block, and keep going until your tower is as tall as you want it to be.
In the same way, atoms can also bond together to form molecules. In the case of diphosphane, the two phosphorus atoms are bonding together to form one molecule, just like stacking blocks together to make a tower.
But wait, there's more! Diphosphane is actually a gas at room temperature and pressure. This means that if you were to try to pick it up with your hands, you wouldn't be able to because it would just float away.
So, to summarize: diphosphane is a gas made up of two phosphorus atoms and four hydrogen atoms that are bonded together to form a molecule. It's like building a tower out of blocks, but with atoms instead.
Related topics others have asked about:
