Doublet (physics)
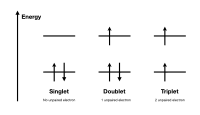
A doublet in physics is a pair of two very close, opposite and equal sources of energy, known as dipoles. Imagine having two little magnets with opposite poles nearby. They would be attracted to each other and form a doublet. Similarly, in physics, when two particles that have opposite charges have an equal amount of energy, they form a doublet.
Doublets are common in many areas of physics such as spectroscopy, where they are used to study and identify chemical elements. Spectroscopists use the light radiation emitted by atoms to determine their composition, and the observed light often comes from doublets. When an atom is excited, it emits specific wavelengths of light which can be seen as spectral lines on a graph. Doublets appear as two very close spectral lines because they come from two sources of opposite charges that contribute equally to the light emission.
For example, the sodium doublet is a set of two very close spectral lines in the yellow part of the visible spectrum, arising from two excited states of sodium atoms. These lines are used to find the presence of sodium in many substances, such as minerals, food, and stars.
Overall, doublets are important because their properties, such as their spacing and intensity, can provide valuable information about the sources, like the chemical composition of the light-emitting object, that generated them.
Doublets are common in many areas of physics such as spectroscopy, where they are used to study and identify chemical elements. Spectroscopists use the light radiation emitted by atoms to determine their composition, and the observed light often comes from doublets. When an atom is excited, it emits specific wavelengths of light which can be seen as spectral lines on a graph. Doublets appear as two very close spectral lines because they come from two sources of opposite charges that contribute equally to the light emission.
For example, the sodium doublet is a set of two very close spectral lines in the yellow part of the visible spectrum, arising from two excited states of sodium atoms. These lines are used to find the presence of sodium in many substances, such as minerals, food, and stars.
Overall, doublets are important because their properties, such as their spacing and intensity, can provide valuable information about the sources, like the chemical composition of the light-emitting object, that generated them.
Related topics others have asked about:
