Dry cell
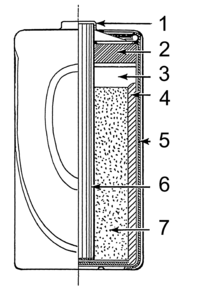
A dry cell is a type of battery that we use to make things like flashlights, toys, and radios work. It looks like a little cylinder with a positive (+) and negative (-) end.
Inside the dry cell are different parts that help produce the electricity. The main two parts are the cathode and anode. The cathode is the positive end and the anode is the negative end.
There are also chemicals inside the dry cell that help create the electricity. These chemicals include things like zinc and manganese dioxide.
When we connect a dry cell to something like a flashlight, the chemicals inside react with each other to produce electricity. This electricity flows through wires inside the flashlight and powers the lightbulb.
Dry cells are called "dry" because they don't have any liquid inside like some other types of batteries. This makes them less messy and easier to use. They also tend to last longer than other types of batteries, which is why we use them in so many everyday things.
Inside the dry cell are different parts that help produce the electricity. The main two parts are the cathode and anode. The cathode is the positive end and the anode is the negative end.
There are also chemicals inside the dry cell that help create the electricity. These chemicals include things like zinc and manganese dioxide.
When we connect a dry cell to something like a flashlight, the chemicals inside react with each other to produce electricity. This electricity flows through wires inside the flashlight and powers the lightbulb.
Dry cells are called "dry" because they don't have any liquid inside like some other types of batteries. This makes them less messy and easier to use. They also tend to last longer than other types of batteries, which is why we use them in so many everyday things.
Related topics others have asked about:
