Endergonic reaction
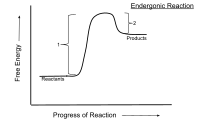
Okay kiddo, let's talk about endergonic reactions.
First, we need to understand that chemical reactions happen when two or more things come together and create something new. And sometimes, these reactions require energy to happen.
Now, an endergonic reaction is a type of reaction that requires MORE energy to happen than it releases. Imagine you're trying to build a tower out of blocks. You have to put in energy to stack the blocks higher and higher, but eventually, the tower will become unstable and tumble down. In a way, that's like an endergonic reaction.
In the world of science, we use a lot of fancy words to describe these reactions. One of these words is "endergonic". It basically means that the reaction needs more energy put into it than it can give back.
Some examples of endergonic reactions include photosynthesis, where plants use energy from the sun to create food. Another example is the process of building muscle. When you lift weights, you're putting energy into your muscles, and they grow as a result.
So, in summary, an endergonic reaction is a chemical reaction that needs more energy put into it than it can give back. It's like building a tower out of blocks that gets too tall and falls over. But sometimes, these reactions are necessary for life processes like growing plants and building muscle.
First, we need to understand that chemical reactions happen when two or more things come together and create something new. And sometimes, these reactions require energy to happen.
Now, an endergonic reaction is a type of reaction that requires MORE energy to happen than it releases. Imagine you're trying to build a tower out of blocks. You have to put in energy to stack the blocks higher and higher, but eventually, the tower will become unstable and tumble down. In a way, that's like an endergonic reaction.
In the world of science, we use a lot of fancy words to describe these reactions. One of these words is "endergonic". It basically means that the reaction needs more energy put into it than it can give back.
Some examples of endergonic reactions include photosynthesis, where plants use energy from the sun to create food. Another example is the process of building muscle. When you lift weights, you're putting energy into your muscles, and they grow as a result.
So, in summary, an endergonic reaction is a chemical reaction that needs more energy put into it than it can give back. It's like building a tower out of blocks that gets too tall and falls over. But sometimes, these reactions are necessary for life processes like growing plants and building muscle.
Related topics others have asked about:
