Gauche effect
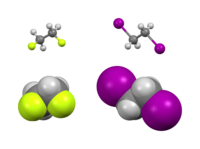
So you know how sometimes you hold your hands to your sides, and sometimes you hold one hand on your hip? Well, atoms in molecules can also have preferred positions that they like to be in.
Some molecules have a "gauche effect," which means that certain atoms in the molecule would rather be in certain positions next to each other, kind of like how you might prefer to stand with one hand on your hip instead of both hands to your sides.
This can happen when two groups of atoms are attached to a central atom and they are next to each other. They like to be in specific positions relative to each other - like one group on the left and the other group on the right. If they are in any other position, it can cause some energy to be lost as the molecule tries to wiggle around to get back to its preferred positions.
So basically, the gauche effect is when certain groups of atoms in a molecule like to be in specific positions relative to each other, and being in any other position can cause the molecule to lose energy.
Some molecules have a "gauche effect," which means that certain atoms in the molecule would rather be in certain positions next to each other, kind of like how you might prefer to stand with one hand on your hip instead of both hands to your sides.
This can happen when two groups of atoms are attached to a central atom and they are next to each other. They like to be in specific positions relative to each other - like one group on the left and the other group on the right. If they are in any other position, it can cause some energy to be lost as the molecule tries to wiggle around to get back to its preferred positions.
So basically, the gauche effect is when certain groups of atoms in a molecule like to be in specific positions relative to each other, and being in any other position can cause the molecule to lose energy.
Related topics others have asked about:
