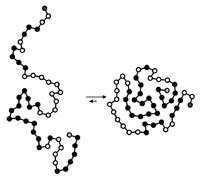
Hydrophobic collapse
Okay, kiddo, have you ever played with magnets before? You know how the opposite poles of magnets attract each other and the same poles repel each other, right? Well, some things in nature behave similarly to magnets - like water molecules.
Water molecules are a bit like tiny magnets. The part of the molecule with the oxygen atom has a slight negative charge, while the hydrogens have a slight positive charge. This makes water molecules attract each other, forming a kind of sticky web that we call a hydrogen bond.
Now imagine you have a long string of beads. If you dip it into water, the beads will be surrounded by water molecules sticking to them because of hydrogen bonds. But what if we had some beads covered with something that water molecule don't like, like oil?
That's where hydrophobic collapse comes in. The beads covered with oil will repel water molecules and push them away. This will cause the water molecules near the beads to become more agitated and restless because they don't like being pushed away from the beads.
Over time, the water molecules will rearrange themselves in a way that minimizes the area of oil exposed to the water. This is called the hydrophobic collapse - the water molecules will fold and organize themselves tightly around the oily beads in a way that minimizes the contact between water and oil. This process is essential for the proper folding of proteins, which are made up of individual amino acids that are hydrophilic (water-loving) or hydrophobic (water-hating).
In fact, without hydrophobic collapse, DNA and many other biological molecules wouldn't work properly! So even though it might seem a little strange, this process is incredibly important for life as we know it.
Water molecules are a bit like tiny magnets. The part of the molecule with the oxygen atom has a slight negative charge, while the hydrogens have a slight positive charge. This makes water molecules attract each other, forming a kind of sticky web that we call a hydrogen bond.
Now imagine you have a long string of beads. If you dip it into water, the beads will be surrounded by water molecules sticking to them because of hydrogen bonds. But what if we had some beads covered with something that water molecule don't like, like oil?
That's where hydrophobic collapse comes in. The beads covered with oil will repel water molecules and push them away. This will cause the water molecules near the beads to become more agitated and restless because they don't like being pushed away from the beads.
Over time, the water molecules will rearrange themselves in a way that minimizes the area of oil exposed to the water. This is called the hydrophobic collapse - the water molecules will fold and organize themselves tightly around the oily beads in a way that minimizes the contact between water and oil. This process is essential for the proper folding of proteins, which are made up of individual amino acids that are hydrophilic (water-loving) or hydrophobic (water-hating).
In fact, without hydrophobic collapse, DNA and many other biological molecules wouldn't work properly! So even though it might seem a little strange, this process is incredibly important for life as we know it.
