Iron oxide cycle
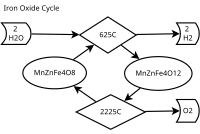
Iron oxide is like a magic rock that changes color when it gets hot or cold. When iron oxide gets heated up, it turns a reddish-brown color, and when it cools down, it turns dark gray. This cycle happens naturally all the time in the world around us!
Let's imagine we have a big pile of iron oxide rocks. If we heat up the pile, the rocks will start to turn red-brown. This is called the "reduction" phase, because the iron oxide is losing oxygen and becoming pure iron.
Once the rocks are hot and red-brown, we can add more oxygen, and the rocks will start to cool down and turn dark gray again. This is called the "oxidation" phase, because the iron is combining with oxygen and becoming iron oxide again.
This cycle can happen naturally in the world, too. For example, when a piece of iron is left outside, it can rust over time. Rust is another name for iron oxide, and it forms when iron combines with oxygen over a long period of time.
The iron oxide cycle is important for many different things in the world, like how we make steel and other metals. Understanding how it works helps us make things better and more efficiently, which is great for us all!
Let's imagine we have a big pile of iron oxide rocks. If we heat up the pile, the rocks will start to turn red-brown. This is called the "reduction" phase, because the iron oxide is losing oxygen and becoming pure iron.
Once the rocks are hot and red-brown, we can add more oxygen, and the rocks will start to cool down and turn dark gray again. This is called the "oxidation" phase, because the iron is combining with oxygen and becoming iron oxide again.
This cycle can happen naturally in the world, too. For example, when a piece of iron is left outside, it can rust over time. Rust is another name for iron oxide, and it forms when iron combines with oxygen over a long period of time.
The iron oxide cycle is important for many different things in the world, like how we make steel and other metals. Understanding how it works helps us make things better and more efficiently, which is great for us all!
Related topics others have asked about:
