Isolobal principle
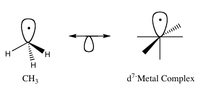
Okay kiddo, imagine you have two toys - a blue ball and a green block. They look very different, right? But what if I told you that they are actually kind of the same?
This is kind of like the isolobal principle. It's a science idea that says two things can be considered the same if they have the same number of electrons and the same number of atoms, even if they look different.
Just like how the blue ball and green block are different shapes, things that are isolobal might look different from each other too. But they still have something important in common - their electrons and atoms.
Scientists use the isolobal principle to help them make new molecules and materials. By understanding how two different things can be isolobal, they can figure out how to put them together to make something new and useful.
So remember - even if things look different, they can still be the same on the inside!
This is kind of like the isolobal principle. It's a science idea that says two things can be considered the same if they have the same number of electrons and the same number of atoms, even if they look different.
Just like how the blue ball and green block are different shapes, things that are isolobal might look different from each other too. But they still have something important in common - their electrons and atoms.
Scientists use the isolobal principle to help them make new molecules and materials. By understanding how two different things can be isolobal, they can figure out how to put them together to make something new and useful.
So remember - even if things look different, they can still be the same on the inside!
