Microscale thermophoresis
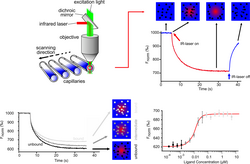
Microscale thermophoresis is a big word, but it's actually not that complicated to understand. Imagine you have two different types of candy, like Skittles and M&Ms, and you want to see which candy the ants in your backyard like more. One way to figure that out is by putting some Skittles and M&Ms in a bowl, pouring hot water on top, and looking at how the ants move towards one candy or the other.
Now imagine that instead of ants and candies, we are trying to figure out how different tiny molecules in our body, like proteins or DNA, interact with each other. We can't pour hot water on them, obviously, so we use a special technique called microscale thermophoresis.
Basically, we take two different types of molecules and label them each with a different color, like red and green. Then we put them in a tiny tube with a heater at one end and a detector at the other end. When we heat up the liquid, the molecules start moving, and we can measure how much the red and green molecules move towards or away from the heat source using the detector.
The distance and speed at which the molecules move will depend on how strongly they interact with each other, like magnets with different strengths. So by measuring how the two molecules move in response to heat, we can tell whether or not they like each other, and how much.
This information is really valuable for scientists who are trying to understand how different molecules in our body work and how they might be malfunctioning in diseases. For example, if we discover that two proteins that are supposed to work together in a healthy cell don't interact as much as they should, we might be able to design a drug that helps them get back together and do their job properly.
Microscale thermophoresis is a powerful tool that helps scientists discover how the tiny building blocks of life interact with each other, and it's kind of like watching candy and ants in your backyard, but at a much smaller scale.
Now imagine that instead of ants and candies, we are trying to figure out how different tiny molecules in our body, like proteins or DNA, interact with each other. We can't pour hot water on them, obviously, so we use a special technique called microscale thermophoresis.
Basically, we take two different types of molecules and label them each with a different color, like red and green. Then we put them in a tiny tube with a heater at one end and a detector at the other end. When we heat up the liquid, the molecules start moving, and we can measure how much the red and green molecules move towards or away from the heat source using the detector.
The distance and speed at which the molecules move will depend on how strongly they interact with each other, like magnets with different strengths. So by measuring how the two molecules move in response to heat, we can tell whether or not they like each other, and how much.
This information is really valuable for scientists who are trying to understand how different molecules in our body work and how they might be malfunctioning in diseases. For example, if we discover that two proteins that are supposed to work together in a healthy cell don't interact as much as they should, we might be able to design a drug that helps them get back together and do their job properly.
Microscale thermophoresis is a powerful tool that helps scientists discover how the tiny building blocks of life interact with each other, and it's kind of like watching candy and ants in your backyard, but at a much smaller scale.
