Quantum numbers
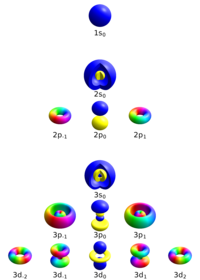
Imagine you have a toy box full of different toys. Each toy can be identified by its different characteristics like color, shape, size, and kind of material. Similarly, in the world of atoms, we have electrons that also have different characteristics that identify them known as quantum numbers.
The first quantum number is called the principal quantum number (n), which is like the number of levels of your toy box. The bigger this number is, the bigger the orbit of the electron is within the atom.
The second quantum number is the angular momentum quantum number (l), which is like the shape of your toy. Here instead of shapes like squares, triangles, and circles, we have orbitals with shapes like s, p, d, f, and so on.
The third quantum number is the magnetic quantum number (m), which is like pointing to which side of the toy box the toy is in. In this case, we indicate the location of the electron inside the orbitals.
Lastly, we have the spin quantum number (s), which is like spinning the toy around its axis. Electrons can only spin in two ways, clockwise or counterclockwise.
All of these quantum numbers help us understand the different types of electrons in an atom and their behavior.
The first quantum number is called the principal quantum number (n), which is like the number of levels of your toy box. The bigger this number is, the bigger the orbit of the electron is within the atom.
The second quantum number is the angular momentum quantum number (l), which is like the shape of your toy. Here instead of shapes like squares, triangles, and circles, we have orbitals with shapes like s, p, d, f, and so on.
The third quantum number is the magnetic quantum number (m), which is like pointing to which side of the toy box the toy is in. In this case, we indicate the location of the electron inside the orbitals.
Lastly, we have the spin quantum number (s), which is like spinning the toy around its axis. Electrons can only spin in two ways, clockwise or counterclockwise.
All of these quantum numbers help us understand the different types of electrons in an atom and their behavior.
Related topics others have asked about:
