Silicene
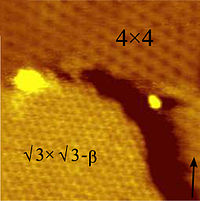
Silicene is like a cousin of graphene, which is a super-strong and super-thin material that is made of carbon atoms. Instead of carbon, silicene is made of silicon atoms, which is a chemical element that is often used in computer chips.
Like graphene, silicene is incredibly thin - it's just one atom thick. But unlike graphene, silicene has a unique honeycomb structure. This means that the silicon atoms are arranged in a pattern that looks like a honeycomb.
Silicene has many potential uses because of its unique properties. For example, it could be used in future nanoelectronics because it can carry electrons very efficiently. It could also be used to make faster and more efficient computer chips because it is very flexible and can be stretched or compressed to change its properties.
However, scientists are still studying silicene to better understand its properties and how it can be used. So we may have to wait a little longer before we see silicene being used in everyday applications.
Like graphene, silicene is incredibly thin - it's just one atom thick. But unlike graphene, silicene has a unique honeycomb structure. This means that the silicon atoms are arranged in a pattern that looks like a honeycomb.
Silicene has many potential uses because of its unique properties. For example, it could be used in future nanoelectronics because it can carry electrons very efficiently. It could also be used to make faster and more efficient computer chips because it is very flexible and can be stretched or compressed to change its properties.
However, scientists are still studying silicene to better understand its properties and how it can be used. So we may have to wait a little longer before we see silicene being used in everyday applications.
