Solvation shell
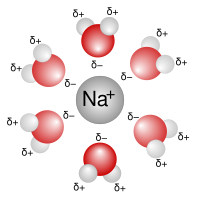
Alright kiddo, let's talk about something called solvation shell. You know how when you put some salt in water and it seems to disappear? Well, what's actually happening is that the salt is dissolving in the water. And when the salt dissolves, it gets surrounded by water molecules.
This group of water molecules is called a solvation shell. It's like a cozy little blanket of water surrounding the salt. The water molecules in the solvation shell are holding on to the salt ions because they have an electric charge. The positive sodium ions are attracted to the negative oxygen atoms in the water, while the negative chloride ions are attracted to the positive hydrogen atoms in the water.
The solvation shell is really important in chemical reactions because it can affect how fast and how easily the reaction happens. It can also change the properties of the solute (the salt in this case) because it's interacting with the solvent (the water) in a very specific way.
So, in short, solvation shell is a group of water molecules that surround a dissolved substance and interact with it in a specific way.
This group of water molecules is called a solvation shell. It's like a cozy little blanket of water surrounding the salt. The water molecules in the solvation shell are holding on to the salt ions because they have an electric charge. The positive sodium ions are attracted to the negative oxygen atoms in the water, while the negative chloride ions are attracted to the positive hydrogen atoms in the water.
The solvation shell is really important in chemical reactions because it can affect how fast and how easily the reaction happens. It can also change the properties of the solute (the salt in this case) because it's interacting with the solvent (the water) in a very specific way.
So, in short, solvation shell is a group of water molecules that surround a dissolved substance and interact with it in a specific way.
Related topics others have asked about:
