Atomic theory
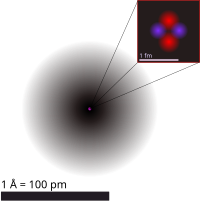
Atomic theory can be explained like building blocks. Imagine you have a box of colorful LEGO blocks. Each block is like an atom. Atoms are really tiny things that make up everything in the world around us, like you, the trees, and even the air we breathe.
Atoms are made up of three tiny particles called protons, neutrons, and electrons. These particles are like the different shapes and sizes of LEGO blocks you have in your box. Protons and neutrons are found in the center of an atom, called the nucleus. Electrons are outside the nucleus, moving around the center like they are playing tag.
Just like you can create different things by putting different LEGO blocks together in different ways, scientists have discovered that different atoms can be created by putting different numbers of protons, neutrons, and electrons together in different ways. For example, the atoms that make up the air we breathe have different combinations of these particles than the atoms that make up metal, like the coins in your piggy bank.
Atomic theory helps scientists understand how atoms work and how they combine to create everything in the world around us. Just like you can use your LEGO blocks to build cars, houses, and even spaceships, scientists can use the knowledge they have about atomic theory to create new materials and technologies that help us live better and more complex lives.
Atoms are made up of three tiny particles called protons, neutrons, and electrons. These particles are like the different shapes and sizes of LEGO blocks you have in your box. Protons and neutrons are found in the center of an atom, called the nucleus. Electrons are outside the nucleus, moving around the center like they are playing tag.
Just like you can create different things by putting different LEGO blocks together in different ways, scientists have discovered that different atoms can be created by putting different numbers of protons, neutrons, and electrons together in different ways. For example, the atoms that make up the air we breathe have different combinations of these particles than the atoms that make up metal, like the coins in your piggy bank.
Atomic theory helps scientists understand how atoms work and how they combine to create everything in the world around us. Just like you can use your LEGO blocks to build cars, houses, and even spaceships, scientists can use the knowledge they have about atomic theory to create new materials and technologies that help us live better and more complex lives.
Related topics others have asked about:
