Azeotropic distillation
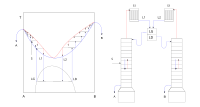
Ok kiddo, let's imagine that we have a glass of orange juice with pulp and you want to separate the juice from the pulp. One way to do it is by using a strainer, right? But what if the pulp and the juice are mixed so well that it's hard to separate them with a strainer? That's where azeotropic distillation comes in.
Azeotropic distillation is like a fancy straining method for liquids that are mixed together so well that straining won't work. Imagine we have two liquids that are mixed together and one of them is water. We want to separate the other liquid from the water but they have similar boiling points and they keep evaporating together.
So, azeotropic distillation works by adding another liquid into the mix. This third liquid has a lower boiling point than the other two liquids. Let's call it "helper liquid". The three liquids are then heated together and the helper liquid helps to evaporate the other two liquids at a temperature lower than their normal boiling points.
As the steam from the mixture rises, the condenser cools it down and it turns back into a liquid. The condensed mixture is collected in a separate container. This way the two liquids are separated and we get a pure sample of the one we're interested in.
In our orange juice example, instead of straining the juice and the pulp, we could add a third liquid like alcohol to the mix. We heat the mixture and as the alcohol evaporates, it takes the juice with it while leaving the pulp behind. Then we cool down the steam and we get a clear orange juice without pulp.
So, that's how azeotropic distillation works. It's like using a fancy straining method for liquids that are mixed together too well to be separated by other means.
Azeotropic distillation is like a fancy straining method for liquids that are mixed together so well that straining won't work. Imagine we have two liquids that are mixed together and one of them is water. We want to separate the other liquid from the water but they have similar boiling points and they keep evaporating together.
So, azeotropic distillation works by adding another liquid into the mix. This third liquid has a lower boiling point than the other two liquids. Let's call it "helper liquid". The three liquids are then heated together and the helper liquid helps to evaporate the other two liquids at a temperature lower than their normal boiling points.
As the steam from the mixture rises, the condenser cools it down and it turns back into a liquid. The condensed mixture is collected in a separate container. This way the two liquids are separated and we get a pure sample of the one we're interested in.
In our orange juice example, instead of straining the juice and the pulp, we could add a third liquid like alcohol to the mix. We heat the mixture and as the alcohol evaporates, it takes the juice with it while leaving the pulp behind. Then we cool down the steam and we get a clear orange juice without pulp.
So, that's how azeotropic distillation works. It's like using a fancy straining method for liquids that are mixed together too well to be separated by other means.
Related topics others have asked about:
