Boltzmann equation
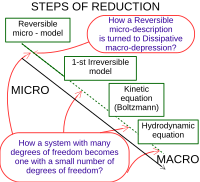
The Boltzmann equation explains how the temperature of a gas is related to the energy of its particles. It's made up of the equation E = kT, where E is the energy of the particles, k is a constant, and T is the temperature. The equation says that the higher the temperature of the gas, the higher the energy of its particles will be. To explain it simply, the Boltzmann equation tells us that when something gets hotter, its particles move around faster and faster!
Related topics others have asked about:
