Chemical polarity
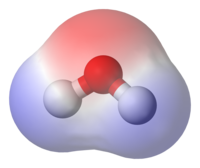
Polarity is a measure of how evenly something is "split" between two different parts. In the case of chemicals, it means how evenly the positive and negative charges in the molecule are spread out. If a molecule is equally split between positive and negative, then it has no polarity, or is non-polar. If the positive and negative charges are not evenly split, then the molecule is considered to be polar.
Related topics others have asked about:
