Fuel cell
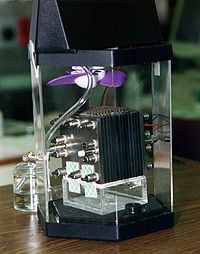
A fuel cell is like a battery that uses energy stored in a fuel such as hydrogen to produce electricity with no pollution. It is like a battery in that it has two electrodes, an anode and a cathode, and a substance called an electrolyte between them. But instead of charging with electricity like a battery, a fuel cell is charged with a fuel like hydrogen. Hydrogen is put into the anode and air is put into the cathode. The hydrogen is then broken down into electrons and protons that can move through the electrodes and the electrolyte, creating an electric current and producing energy. The only by-product is water!
Related topics others have asked about:
