Ideal gas laws
Hey kiddo, have you ever blown up a balloon? When you blow up a balloon, you're actually filling it with gas - air, to be specific. And did you notice that when you blow up a balloon, it gets bigger and bigger until it's full? That's because the air inside the balloon is pushing out, trying to get more space to spread out.
Now imagine that the balloon is actually a container filled with gas, like helium or oxygen. The same thing happens - the gas particles inside the container are moving around and bouncing off of each other, trying to take up as much space as possible. But what happens when we change things, like the amount of gas inside the container or the temperature outside? That's where the ideal gas laws come in.
There are three main laws that describe how gases behave:
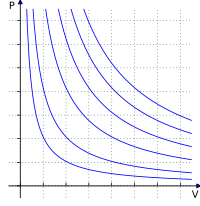
1. The first law is called Boyle's Law. It says that if we keep the temperature of a gas the same, the pressure and volume of the gas are inversely proportional. That means that if we decrease the volume of a gas, the pressure inside will increase. And if we increase the volume of a gas, the pressure inside will decrease. Think of squeezing a balloon - if you make it smaller, the air inside gets squished and the pressure goes up.
2. The second law is called Charles's Law. It says that if we keep the pressure of a gas the same, the volume and temperature of the gas are directly proportional. That means that if we increase the temperature of a gas, the volume will also increase. And if we decrease the temperature, the volume will go down. Think of a balloon again - if you leave it in the hot sun for too long, it will expand because the air inside is getting hotter and taking up more space.
3. The third law is called Avogadro's Law. It says that if we keep the pressure and temperature of a gas the same, the volume and number of particles in the gas are directly proportional. That means that if we add more gas particles to a container, the volume will increase to accommodate them. And if we take away particles, the volume will decrease. This law is like adding more balloons to a room - the more balloons you add, the more space they take up.
So those are the three main ideal gas laws - Boyle's Law, Charles's Law, and Avogadro's Law. They help us understand how gases behave and why they do the things they do. Pretty cool, huh?
Now imagine that the balloon is actually a container filled with gas, like helium or oxygen. The same thing happens - the gas particles inside the container are moving around and bouncing off of each other, trying to take up as much space as possible. But what happens when we change things, like the amount of gas inside the container or the temperature outside? That's where the ideal gas laws come in.
There are three main laws that describe how gases behave:
1. The first law is called Boyle's Law. It says that if we keep the temperature of a gas the same, the pressure and volume of the gas are inversely proportional. That means that if we decrease the volume of a gas, the pressure inside will increase. And if we increase the volume of a gas, the pressure inside will decrease. Think of squeezing a balloon - if you make it smaller, the air inside gets squished and the pressure goes up.
2. The second law is called Charles's Law. It says that if we keep the pressure of a gas the same, the volume and temperature of the gas are directly proportional. That means that if we increase the temperature of a gas, the volume will also increase. And if we decrease the temperature, the volume will go down. Think of a balloon again - if you leave it in the hot sun for too long, it will expand because the air inside is getting hotter and taking up more space.
3. The third law is called Avogadro's Law. It says that if we keep the pressure and temperature of a gas the same, the volume and number of particles in the gas are directly proportional. That means that if we add more gas particles to a container, the volume will increase to accommodate them. And if we take away particles, the volume will decrease. This law is like adding more balloons to a room - the more balloons you add, the more space they take up.
So those are the three main ideal gas laws - Boyle's Law, Charles's Law, and Avogadro's Law. They help us understand how gases behave and why they do the things they do. Pretty cool, huh?
Related topics others have asked about:
