Isoelectronicity
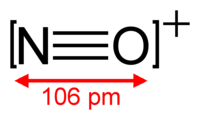
Hey there, did you ever think about how different elements have different numbers of electrons? Well, sometimes elements can have the same number of electrons, even though they are different elements! This is called isoelectronicity.
Imagine that you have a lot of different toys with different colors and shapes. Some toys have 1, 2, 3, or even more pieces to play with. But now imagine that you have different toys that may look different, but they actually have the same number of pieces! That's just like isoelectronic elements.
Elements are made up of tiny things called electrons that move around the nucleus, which is like the heart of the element. Sometimes two or more different elements have the same number of electrons, even though they are different elements with different properties, like colors and shapes.
When elements have the same number of electrons, they have similar chemical properties, just like how different toys with the same number of pieces can be used in similar ways. This means that scientists can use isoelectronic elements to make new compounds and study chemical reactions.
So, in short, isoelectronicity is when different elements have the same number of electrons, even though they look different. This can help scientists understand and predict how different elements will react with each other.
Imagine that you have a lot of different toys with different colors and shapes. Some toys have 1, 2, 3, or even more pieces to play with. But now imagine that you have different toys that may look different, but they actually have the same number of pieces! That's just like isoelectronic elements.
Elements are made up of tiny things called electrons that move around the nucleus, which is like the heart of the element. Sometimes two or more different elements have the same number of electrons, even though they are different elements with different properties, like colors and shapes.
When elements have the same number of electrons, they have similar chemical properties, just like how different toys with the same number of pieces can be used in similar ways. This means that scientists can use isoelectronic elements to make new compounds and study chemical reactions.
So, in short, isoelectronicity is when different elements have the same number of electrons, even though they look different. This can help scientists understand and predict how different elements will react with each other.
Related topics others have asked about:
