Moseley's law
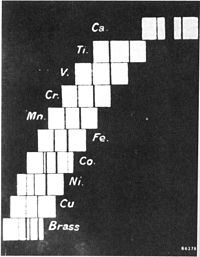
Have you ever seen a number line, like the one you use in math class? In science, we also have something called a number line, but instead of counting numbers, we count things called elements. Elements are like the building blocks of everything around us, like the air we breathe and the water we drink.
Scientists like to study these elements, and they found out that each element has its own special number. They call this number a "atomic number." So, for example, the element hydrogen has an atomic number of 1, while the element helium has an atomic number of 2.
Now, a scientist named Moseley noticed something interesting about these atomic numbers. He found that when he arranged the elements in order of their atomic numbers, some patterns started to emerge. He called this the "periodic law."
But Moseley didn't stop there. He wanted to figure out why these patterns were happening. So, he did some more experiments and found out that the the energy of the atoms depended on their atomic number. He called this the "Moseley's law."
Basically, what this means is that the higher the atomic number of an element, the more energy its atoms have. And this energy can tell scientists a lot about how the elements behave and what they can be used for. So, Moseley's law helps scientists better understand the elements and use them in useful ways.
Scientists like to study these elements, and they found out that each element has its own special number. They call this number a "atomic number." So, for example, the element hydrogen has an atomic number of 1, while the element helium has an atomic number of 2.
Now, a scientist named Moseley noticed something interesting about these atomic numbers. He found that when he arranged the elements in order of their atomic numbers, some patterns started to emerge. He called this the "periodic law."
But Moseley didn't stop there. He wanted to figure out why these patterns were happening. So, he did some more experiments and found out that the the energy of the atoms depended on their atomic number. He called this the "Moseley's law."
Basically, what this means is that the higher the atomic number of an element, the more energy its atoms have. And this energy can tell scientists a lot about how the elements behave and what they can be used for. So, Moseley's law helps scientists better understand the elements and use them in useful ways.
Related topics others have asked about:
