Pre-clinical development
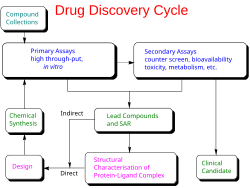
Pre-clinical development is when scientists and researchers do experiments and tests on a new medicine or treatment to see if it might work well in humans. Before a medicine can be tested on people, it needs to go through many tests and experiments to make sure it is safe and effective.
First, researchers do tests on cells in a lab. They might also use animals like rats or mice to see how the medicine affects them. They want to make sure that the medicine doesn't harm the cells or animals, and that it actually does something to help them.
Then, if the medicine seems safe and useful, researchers will do more tests in animals to see if it works well for the illness they are studying. They might test how the medicine is absorbed by the animal's body, how it's processed, and how long it stays in the body.
If the medicine passes all of these tests and shows promise, then it might move on to human testing. This is where people are given the medicine to see how well it works and to make sure that it is safe for humans.
Overall, pre-clinical development is an important process that helps ensure that new medicines and treatments are safe and effective for people to use.
First, researchers do tests on cells in a lab. They might also use animals like rats or mice to see how the medicine affects them. They want to make sure that the medicine doesn't harm the cells or animals, and that it actually does something to help them.
Then, if the medicine seems safe and useful, researchers will do more tests in animals to see if it works well for the illness they are studying. They might test how the medicine is absorbed by the animal's body, how it's processed, and how long it stays in the body.
If the medicine passes all of these tests and shows promise, then it might move on to human testing. This is where people are given the medicine to see how well it works and to make sure that it is safe for humans.
Overall, pre-clinical development is an important process that helps ensure that new medicines and treatments are safe and effective for people to use.
Related topics others have asked about:
