Stereocenter
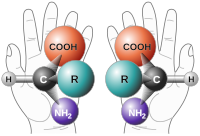
Imagine you have a toy car with four different colored wheels: red, yellow, green, and blue. Now let's say you want to make another car that looks exactly the same as the first one, but with one small difference - you want to make the green wheel face the opposite direction.
To do that, you need to take the green wheel off the car and turn it around, so that the side that was facing forward is now facing backward. This is pretty simple, right?
Now let's apply this concept to chemistry. In a molecule, there are four different "arms" sticking out of a central atom. These arms can be different atoms or groups of atoms, like hydrogen, oxygen, or carbon. When all four arms are different, we call this atom a stereocenter.
Just like with the toy car and the green wheel, you can imagine rotating these arms in certain ways to create different versions of the same molecule. These different versions are called stereoisomers, and they can have different properties, like taste, odor, or even biological activity.
So why is this important in real life? Well, scientists and doctors need to understand the structure of molecules to develop new drugs, for example. By looking at the different stereoisomers of a molecule, they can determine which one is the most effective or safest for treating a certain condition.
Overall, stereocenters are an important concept in chemistry that help us understand how molecules behave and how we can manipulate them to make new materials or medicines.
To do that, you need to take the green wheel off the car and turn it around, so that the side that was facing forward is now facing backward. This is pretty simple, right?
Now let's apply this concept to chemistry. In a molecule, there are four different "arms" sticking out of a central atom. These arms can be different atoms or groups of atoms, like hydrogen, oxygen, or carbon. When all four arms are different, we call this atom a stereocenter.
Just like with the toy car and the green wheel, you can imagine rotating these arms in certain ways to create different versions of the same molecule. These different versions are called stereoisomers, and they can have different properties, like taste, odor, or even biological activity.
So why is this important in real life? Well, scientists and doctors need to understand the structure of molecules to develop new drugs, for example. By looking at the different stereoisomers of a molecule, they can determine which one is the most effective or safest for treating a certain condition.
Overall, stereocenters are an important concept in chemistry that help us understand how molecules behave and how we can manipulate them to make new materials or medicines.
Related topics others have asked about:
