Valence electron
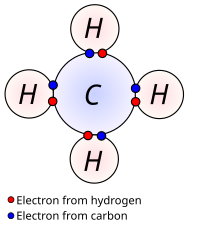
Valence electrons are like the super special friends that atoms have. They are the electrons that are found in the outermost shell or level of the atom. Think of an atom like a little planet with different layers of people living on it, and the valence electrons are like the cool kids who live on the outer layer.
Valence electrons are important because they determine how atoms will interact with each other. When atoms get together to form compounds, they share, give away or take on electrons to fill up their outermost layer of people. This helps them become more stable and happy (just like friends who like to stick together when they have fun).
Atoms can have different numbers of valence electrons. For example, Hydrogen has one valence electron, Carbon has four valence electrons, and Oxygen has six valence electrons. Scientists use the number of valence electrons to help figure out how atoms will bond with each other to form molecules and compounds.
So, in simple terms, valence electrons are special electrons that help atoms bond with each other to form cool things like water, sugar or even your body!
Valence electrons are important because they determine how atoms will interact with each other. When atoms get together to form compounds, they share, give away or take on electrons to fill up their outermost layer of people. This helps them become more stable and happy (just like friends who like to stick together when they have fun).
Atoms can have different numbers of valence electrons. For example, Hydrogen has one valence electron, Carbon has four valence electrons, and Oxygen has six valence electrons. Scientists use the number of valence electrons to help figure out how atoms will bond with each other to form molecules and compounds.
So, in simple terms, valence electrons are special electrons that help atoms bond with each other to form cool things like water, sugar or even your body!
