Alkane
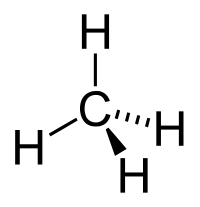
An alkane is like a big chain made up of tiny pieces called atoms. The atoms that make up an alkane are called carbon and hydrogen atoms. Carbon atoms are like Legos and can link together to form very long chains. You can think of the carbon atoms as the links in a chain and the hydrogen atoms as the little bumps that stick out of the chain.
Alkanes can have different lengths depending on how many carbon atoms are in the chain. For example, if there are four carbon atoms, it's called butane. If there are five carbon atoms, it's called pentane. The names of alkanes end with "ane".
Alkanes are known for being very stable, which means they don't like to react easily with other things. This is because their carbon and hydrogen atoms are already bonded together tightly. That's why alkanes are often used as fuels, because they can burn easily to create energy.
In summary, an alkane is a big chain made up of carbon and hydrogen atoms that are linked together. They come in different lengths and are known for being stable and good at creating energy when they burn.
Alkanes can have different lengths depending on how many carbon atoms are in the chain. For example, if there are four carbon atoms, it's called butane. If there are five carbon atoms, it's called pentane. The names of alkanes end with "ane".
Alkanes are known for being very stable, which means they don't like to react easily with other things. This is because their carbon and hydrogen atoms are already bonded together tightly. That's why alkanes are often used as fuels, because they can burn easily to create energy.
In summary, an alkane is a big chain made up of carbon and hydrogen atoms that are linked together. They come in different lengths and are known for being stable and good at creating energy when they burn.
Related topics others have asked about:
