Cycloalkane
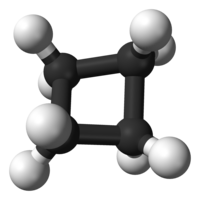
Imagine a bunch of toy balls that are all joined together by sticks, like a string of beads. Now imagine bending that string of balls into a round shape, like a rubber band. That's what a cycloalkane looks like - it's a group of carbon atoms that are arranged in a circular shape, with each carbon atom joined to two other carbon atoms by sticks.
The special thing about cycloalkanes is that they are part of a bigger group of molecules called alkanes. Alkanes are made up of just carbon and hydrogen atoms, and they are known for being very stable and unreactive. Cycloalkanes are just like alkanes, but they have a circular shape instead of a straight line shape.
Now, why is this important? Well, cycloalkanes are found in lots of different things we use every day. They can be found in the fuels we use to power cars and planes, in the plastics we use to make toys and storage containers, and even in the oils we use to cook our food. Understanding how cycloalkanes work and how they interact with other molecules can help us make better and safer products for ourselves and the environment.
The special thing about cycloalkanes is that they are part of a bigger group of molecules called alkanes. Alkanes are made up of just carbon and hydrogen atoms, and they are known for being very stable and unreactive. Cycloalkanes are just like alkanes, but they have a circular shape instead of a straight line shape.
Now, why is this important? Well, cycloalkanes are found in lots of different things we use every day. They can be found in the fuels we use to power cars and planes, in the plastics we use to make toys and storage containers, and even in the oils we use to cook our food. Understanding how cycloalkanes work and how they interact with other molecules can help us make better and safer products for ourselves and the environment.
Related topics others have asked about:
